Inclusion-body myositis is a rare muscular disease that results in muscular weakness. The mechanisms that cause it are not known, and there are no biomarkers or effective treatments available. “One of the main problems that we face is a lack of adequate models for studying the disease” explains Judit Cantó-Santos, a researcher from the IDIBAPS Hereditary metabolic diseases and muscular diseases group led by Glòria Garrabou. Cantó-Santos is the first-signing author of a study, published by the Journal of Cachexia, Sarcopenia and Muscle, that evaluates a cellular model to establish whether it reproduces the characteristic alterations that the disease induces in muscular tissue.
In their work, the researchers analyse the gene expression of fibroblast cultures, obtained from patients with inclusion-body myositis and healthy individuals. “Although the disease affects muscle, in prior studies we have seen that the pathological changes due to myositis are also observed in other cellular types, such as fibroblasts,” the researcher explains. “Fibroblasts are characteristic cells of connective tissue, such as skin, ligaments, cartilage, or adipose tissue. Their proliferation capacity and easy obtention via skin biopsy, as well as a low maintenance cost, makes them an interesting model for various diseases.”
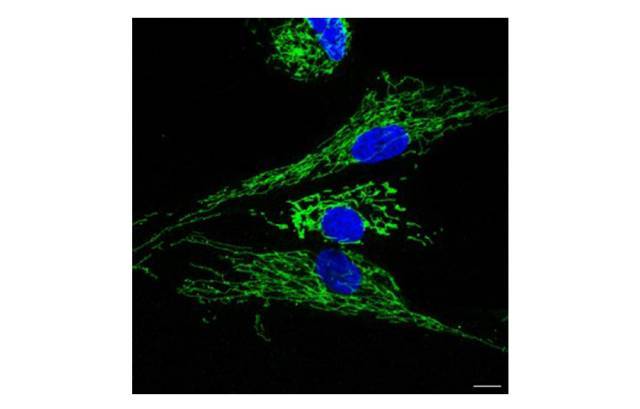
According to the results, fibroblasts derived from patients present alterations in 778 genes, involved in inflammatory, degenerative, and mitochondrial processes. These modifications are very similar to those observed on a muscular level in people affected by the disease. “Furthermore, we have also discovered an increase in the secretion of pro-inflammatory molecules called cytokines, in fibroblast cultures coinciding with the inflammation that takes place in the muscles of patients,” states Glòria Garrabou, the study leader, together with Josep Maria Grau-Junyent. The functional alternations are also reflected in the parameters of autophagy, in other words, of cellular and mitochondrial degradation, which indicate how the cell absorbs, produces, and uses energy. “In other words, the model not only reproduces the changes at the gene expression level, but also the functional changes.”
Although the study presents certain limitations, such as a reduced number of patient samples, the researchers conclude that the data validate the model. “Inclusion-body myositis is a rare disease. This makes it difficult to recruit patients, even for a leading referral unit for the disease such as ours,” points out Cantó-Santos. “In addition, even though there may be other cells that reproduce the pathological processes that take place in the muscle more accurately, their maintenance and cost is higher than that of fibroblasts, which reduces their routine use. Therefore, fibroblasts can be a good model for studying the disease, finding out the causes, and seeking biomarkers and new therapeutic targets.”
The work, which has enjoyed the collaboration of the CNAG-CRG and of Sant Joan de Déu, has received funding from the Carlos III Health Institute.
Reference article
Cantó-Santos J, Valls-Roca L, Tobías E, García-García FJ, Guitart-Mampel M, Esteve-Codina A, Martín-Mur B, Casado M, Artuch R, Solsona-Vilarrasa E, Fernández-Checa JC, García-Ruiz C, Rentero C, Enrich C, Moreno-Lozano PJ, Milisenda JC, Cardellach F, Grau-Junyent JM, Garrabou G. Unravelling inclusion body myositis using a patient-derived fibroblast model. Journal of Cachexia Sarcopenia and Muscle. 1 Mar 2023. doi: 10.1002/jcsm.13178.