Parkinson's disease is the most frequent neurodegenerative movement disorder. In most cases, the origin of the disease is unknown. Approximately 10% of patients, however, present genetic mutations that cause onset of the disease. Notable among the abnormal genes is the gene LRRK2, which is responsible for a large proportion of genetic cases.
Now, Rubén Fernández-Santiago and Mario Ezquerra, researchers of the IDIBAPS group Parkinson disease and other neurodegenerative movement disorders: clinical and experimental research, together with Cristina Malagelada, head of the Neurodegeneration and signalling group at Universitat de Barcelona, have led a study, published in the journal Movement Disorders, which analyzed the effect of LRRK2 mutations.
“Our goal is to identify the molecular mechanisms involved in both genetic and sporadic or idiopathic cases in order to develop more effective therapies”, explained Ezquerra.
LRRK2 is a kinase, i.e., an enzyme that regulates other proteins by the addition of a phosphate group, which can activate or deactivate them. “It has been found that G2019S or R1441G mutations of LRRK2 increase the ability of the enzyme to phosphorylate proteins. However, we do not know which proteins LRRK2 act on in clinical conditions, as most studies have been carried out in vitro. Recently, our team analyzed samples from patients with these mutations, as well as carriers of the mutations who have not yet developed the disease, in order to identify these proteins”, continued Ezquerra.
Using mass spectrometry, the researchers detected changes in the phosphorylation pattern of 23 proteins, which make it possible to differentiate Parkinson patients with the G2019S mutation from asymptomatic carriers and healthy people. “The similarity between the profile of people with the mutation who have not yet presented symptoms and healthy individuals may be extremely useful as a biomarker. If the carriers develop the diseases in the future, we would be able to detect the changes in this pattern that would allow us to diagnose and monitor the progress of the Parkinson disease. Furthermore, if we have neuroprotective treatments, we will also be able to see whether they can revert the changes in the phosphorylation profile associated with the disease”, he said.
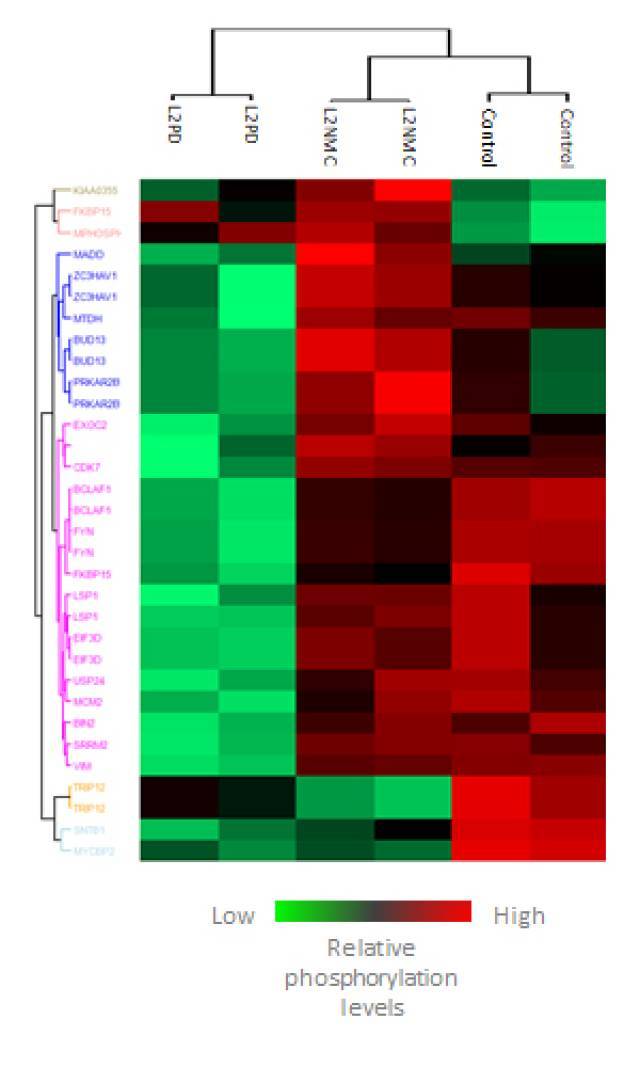
Diagram showing changes in the phosphorylation pattern of 23 proteins in patients with the G2019S mutation (left), asymptomatic carriers (center) and healthy individuals (right).
The researchers also highlight the fact that the abnormal proteins in asymptomatic carriers of the G2019S mutation, in patients with the same mutation, and in patients with idiopathic Parkinson disease or Parkinson disease of unknown cause belong to the same biologic pathway and are involved in neuron survival. “This very likely suggests that both forms of the disease, genetic and idiopathic, share pathologic mechanisms. Thus, the findings in LRRK2 Parkinson disease may also help idiopathic patients, who make up the large majority of cases”, concluded Fernández-Santiago.
This study has received funding from the Young Investors (JIN) program of the Spanish Ministry of Economy and Competitiveness (MINECO) and the Spanish State Research Agency (AEI/FEDER/UE), awarded Rubén Fernández-Santiago (#SAF2015-73508-JIN). The collection of biological samples was funded with the help of the Michael J. Fox Foundation for Parkinson’s Research (MJFF), received by María-José Martí i Eduard Tolosa (#11849). The support of the Michael J. Fox Foundation for Parkinson’s Research has also allowed us to publish the article in an open-access format.
Reference Article
Alicia Garrido, Enrique Santamaría, Joaquín Fernández-Irigoyen, Marta Soto, Cristina Simonet, Manel Fernández, Donina Obiang, Eduardo Tolosa, María-José Martí, Shalini Padmanabhan, Cristina Malagelada, Mario Ezquerra, Rubén Fernández-Santiago. Differential phospho-signatures in blood cells identify LRRK2 G2019S carriers in Parkinson's disease. Mov Disord. 2022 Jan 20. doi: 10.1002/mds.28927




