Neonatal screening has been carried out in Catalonia on more than 3 million babies, of whom 2,500 benefited from this diagnosis and early treatment. Screening is not mandatory, but annually the programme covers 99.9% of newborns.
Neonatal screening provides unquestionable benefits, as it leads to an improvement in the quality of life of patients, allowing them to reach adulthood and have children themselves.
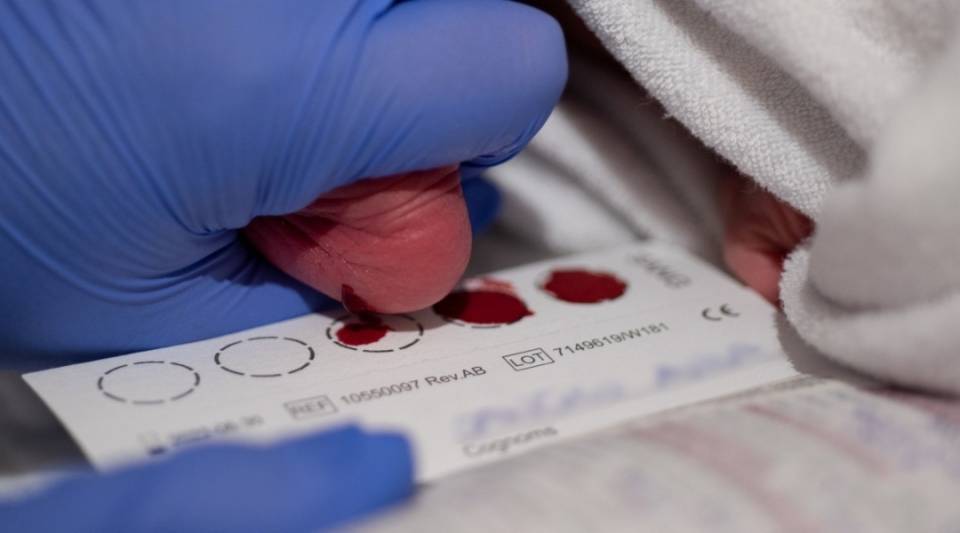
Screening tests are also an advantage due to their simplicity and lack of invasiveness. In maternity centres, a blood spot screening test is performed, by taking blood from the heel of babies of 24-72 hours’ old and impregnating it on a special card, known as heel prick test. The card and a file with the baby's data are sent to the laboratory to analyse for over 60 markers to screen for the 25 diseases included to date. If any of the markers studied is altered, this result is not considered as a diagnosis, but is sent to the Clinical Reference Unit so an appointment can be made with the family for further studies to confirm or rule out the disease.
Screening test evolution
Neonatal screening began in 1969 In Catalonia to help detect phenylketonuria. It was expanded a decade later with screening for congenital hypothyroidism and later with cystic fibrosis.
In 2013, the implementation of a technique using tandem mass spectrometry allowed the inclusion of screening for hereditary metabolic diseases. This multiplied the diseases that could be detected from 3 to 20.
Since then, neonatal screening has continued to expand. In 2015 and 2017, the detection of haemoglobinopathies and severe combined immunodeficiencies were incorporated. The latest inclusion was the detection of biotinidase deficiency in 2022.
Currently, neonatal screening consists mainly of biochemical tests, but this may change with the technological revolution that massive sequencing brings. This technology would allow genetic test screening in the future. This means that the DNA of newborns could be analysed and many more diseases detected, including those without a biochemical marker. However, it would make sense to extend this screening only where early treatment of that disease changes its course. Currently, countries such as the UK and the United States are carrying out pilot studies to analyse the genome of newborns.
Neonatal screening at the Clínic
The neonatal screening laboratory that centralises the analysis of all newborns in Catalonia - around 57,000 per year - is located at the Inborn Errors of Metabolism-IBC Section, Biochemistry and Molecular Genetics Unit, Biomedical Diagnosis Centre at the Hospital Clínic Barcelona. Approximately 160 patients a year are diagnosed early.
The Hospital Clínic neonatal screening laboratory was the first European laboratory to implement the detection of severe combined immunodeficiencies.
The second tier test strategy in Catalonia, using the initial newborn screening sample, has increased the efficiency of the programme, as well as reducing both the number of false positives and babies referred to clinical units for diagnosis to confirm or rule out a disease. The proper functioning of this test has therefore made it possible to reduce the anxiety and stress of families at this very important time in their lives.
INFORMATION DOCUMENTED BY:
Dr Judit García Villoria, Head of the Inborn Errors of Metabolism Section-IBC, Biochemistry and Molecular Genetics Unit.