Chronic lymphocytic leukemia is the most frequent leukemia in Western countries, with more than 12,000 new cases diagnosed in Europe every year. Knowing the genetic alterations present in a tumor is a fundamental step to understand cancer development and improve current treatments.
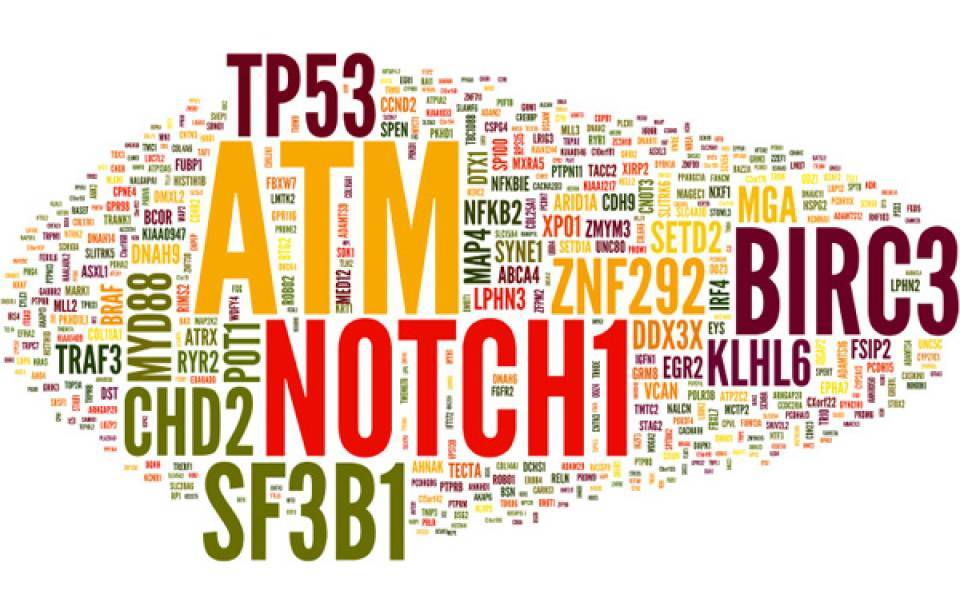
This study shows that each tumor accumulates more than three thousand mutations in its genome, but only a handful of them are relevant for tumor growth. “We have been able to define 60 different genes whose mutations cause tumor initiation and development” comments Dr. Lopez-Otin. “However, the most relevant finding of this study has been the identification of mutations in regions of the genome which do not code for proteins and whose functional relevance is still hardly known”. “These regions represent 98% of our genome, but their functions are poorly understood, so they are not routinely analyzed in patients” explains Dr. Xose S. Puente from the University of Oviedo, and first author of the study. “In this work, we have shown that one in every five tumors originates due to mutations in the so called dark regions of the genome, and knowing this information is essential, as they have a clear impact on the prognosis of the disease”.
The study also reveals the impact of several mutations in the clinical evolution of the patients. “This work provides a comprehensive catalogue of the most important genetic alterations involved in the development of this leukemia”, comments Dr. Elias Campo.
The Spanish Consortium for the Study of Chronic Lymphocytic Leukemia Genome
The Spanish Consortium for the Study of Chronic Lymphocytic Leukemia Genome (www.cllgenome.es) belongs to the International Cancer Genome Consortium (www.icgc.org), led by Tom Hudson from the Ontario Cancer Institute in Toronto. The results generated by this Consortium are providing the grounds for the upcoming use of Precision Medicine initiatives worldwide. Thus, the increasing use of tumor genomic analysis will allow a better classification of patients, as well as better treatment decisions based on the type of genetic alterations present in the tumor. In fact, novel generations of drugs for chronic lymphocytic leukemia are approved for patients with specific genomic alterations, and this situation will become more common as the mutations which determine the response to specific drugs are identified. The study published today confirms the utility of genome sequencing to uncover the genetic causes of cancer and to identify novel mechanisms implicated in its development, as well as to define new therapeutic approaches for its treatment.
More than 15 institutions collaborated on the Chronic Lymphocytic Leukemia Genome Project (www.cllgenome.es), including: Hospital Clinic from Barcelona, Instituto de Oncología Universidad de Oviedo, Institut d’Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, Barcelona Supercomputing Center, Deusto University, Centro Nacional de Análisis Genómico, Hospital Universitario de Salamanca, Hospital Universitario Central de Asturias, Hospital Clínico de Valencia, Institut Català d’Oncologia, Centro de Investigación del Cáncer de Salamanca, Columbia University, NY, USA, Radboud University, Nejmeigen, The Netherlands, Center for Research and Technology, Thesaloniki, Greece, Centre de Regulació Genómica de Barcelona, and Centro Nacional de Investigaciones Oncológicas.
The research was funded by the Spanish Ministerio de Economía y Competitividad through Instituto de Salud Carlos III.
Article reference:
Puente XS, Bea S, Valdes-Mas R et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature, 22 July 2015. DOI: 10.1038/nature14666