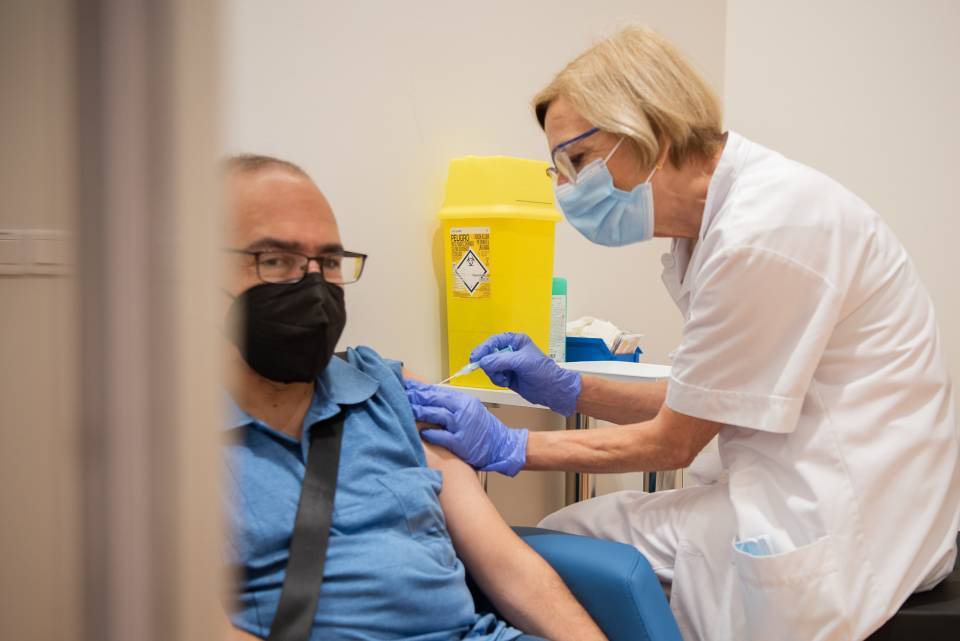
The biotechnological pharmaceutical company HIPRA has received authorization from the Spanish Agency for Medicines and Health Products (AEMPS) to start Phase IIb of the clinical trial of the HIPRA vaccine against Covid-19. The main objectives of the Phase IIb trial are to confirm safety and tolerability for a booster dose and to check if the booster dose prolongs the immune response to Covid-19 in people who have already been vaccinated.
Phase IIb begins after Phase I/IIa, approved by the AEMPS last August, which has demonstrated good tolerability and the absence of significant adverse effects in all participants. This is the first human clinical trial of a vaccine being developed in Spain.
The clinical trial is expected to begin in the next few days in 10 Spanish hospitals: Hospital Clínic de Barcelona (Catalonia), Hospital Universitari Dr. Josep Trueta (Catalonia), Hospital Universitari Vall
d’Hebron (Catalonia), Hospital Germans Trias i Pujol – Can Ruti (Catalonia), Hospital General Universitario Gregorio Marañón (Community of Madrid), Hospital Universitario La Paz (Community of Madrid), Hospital Universitario Príncipe de Asturias (Community of Madrid), Hospital Universitario de Cruces (Basque Country), Hospital Regional Universitario Carlos Haya de Málaga (Andalusia), Hospital Clínico Universitario de Valencia (Valencian Community). It will be carried out with a total of 1,075 volunteers over the age of 18, who have received fully vaccination for the Pfizer-BioNTech Comirnaty vaccine for 6 months and who have not passed the disease.
If the results obtained from Phase IIb are favourable, Phase III will start immediately, including more hospitals in Spain and other European countries and as well as more volunteers. The Covid-19 vaccine that HIPRA is developing is a recombinant protein vaccine that has been designed to optimize its safety and achieve a potent neutralizing immune response to the Covid-19 virus. It will be kept between 2 and 8º C, which will facilitate logistics and distribution.
It is expected that the vaccine will be available in the first half of 2022, subject to obtaining the appropriate
authorizations.