The N-methyl-D-aspartate receptor (NMDAR) is a glutamate receptor, made up of two types of subunits (GluN1 and GluN2), found in nerve cells. This type of receptor plays a key role in neuronal synaptic plasticity and memory function and has been linked to various pathological conditions (e.g. schizophrenia, Parkinson’s disease).
Now, the anti-NMDAR encephalitis is a recently identified severe autoimmune neuropsychiatric disorder characterized by changes in behaviour, psychosis, decrease of memory, seizures, stereotyped movements, autonomic instability, and coma. This disorder is caused by patients’ antibodies, which induce the cross-linking and internalization of NMDA receptors inside the cell, thus altering its function. However, the synaptic events leading to this depletion/internalization of NMDAR are poorly understood.
In a recent study published in Cell Reports, ICFO researchers Laurent Ladépêche, Shreyasi Thakur, Irina Suárez, Joseph Steven Borbely, Angel Sandoval, Lara Laparra, led by Prof. Melike Lakadamyali, in collaboration with IDIBAPS researchers Jesús Planagumà, Makoto Hara, led by Prof. Josep Dalmau, have reported on the nature and timeframe of the processes that take place at the synapse prior to the depletion of NMDA receptors of the cell surface..
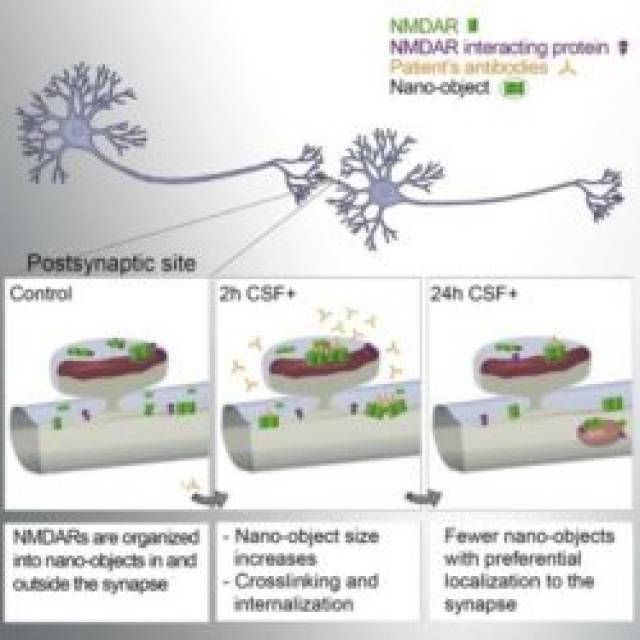
To better understand the mechanisms underlying those observations, the scientists carried out computer simulations to model the behaviour of these receptors along the neuronal dendrites in presence of the patients’ antibodies. They could effectively recapitulate their experimental results, allowing them to extract the key parameters leading to the antibody-induced alterations. They revealed that the disruption of protein-protein interactions, such as the one existing between NMDA and Ephrin B2 receptors, is playing an important role in the sequence of events leading to the disease pathogenesis.
The results of this study shed new light on the nanoscopic processes by which patients’ antibodies can alter the function of a targeted protein, opening a new window into understanding the molecular mechanisms of autoimmune disorders. Future studies aim to determine if other interacting partners with NMDAR could be disturbed by patients’ antibodies in order to discover new potential treatment targets.

