Nearly 30% of the Spanish population suffers from abnormal glucose metabolism, which may lead to severe health problems and complications such as diabetes. “Diabetes is a silent evil. High levels of glucose are often not given enough importance, as they do not produce symptoms. But the effects accumulate over a long time and increase the risk of suffering from severe cardiovascular diseases such as myocardial infarction or stroke, and others such as loss of vision from damage to the retina, damage to the nerves and to the extremities, or kidney failure, which can end in dialysis and kidney transplant”, explained Joan-Marc Servitja, a researcher in the IDIBAPS group Pathogenesis and prevention of diabetes, directed by Anna Novials.
Data show that, in Spain, four-and-a-half million people suffer from diabetes, i.e., almost 14% of the population. “But half of them do not know. It is what we call undiagnosed diabetes”, says Novials. “Despite the importance of these numbers, the money devoted to research is less than that for other diseases such as cancer, Alzheimer, Parkinson, or obesity, even though all of them are closely linked to diabetes. This is of concern to researchers, because we are fighting to raise the profile of the disease and to educate society about its importance”.
One name, several diseases
The word diabetes does not refer to a single disease; it covers several disorders.
Type 1 diabetes is an autoimmune disease characterized by raised levels of glucose in the blood, because the body’s own immune system attacks and destroys the cells of the pancreas that produce insulin.
Type 2 diabetes is also characterized by increased concentrations of glucose in the blood. In this case, however, the cause is the body’s inability to use its own insulin properly. It appears in adults, which is why it was known as adult-onset diabetes and associated with the elderly. This type of diabetes is associated with obesity and a sedentary lifestyle.
“Some 90% of cases of diabetes are type 2. Type 1 diabetes is the second most prevalent form but there is also gestational diabetes and monogenic diabetes or diabetes due to genetic causes, says Servitja. “Indeed, gestational diabetes is increasingly monitored, as it is a sort of warning signal. If the mother has been overweight or suffered from obesity over the years, there is a greater risk of type 2 diabetes appearing”.
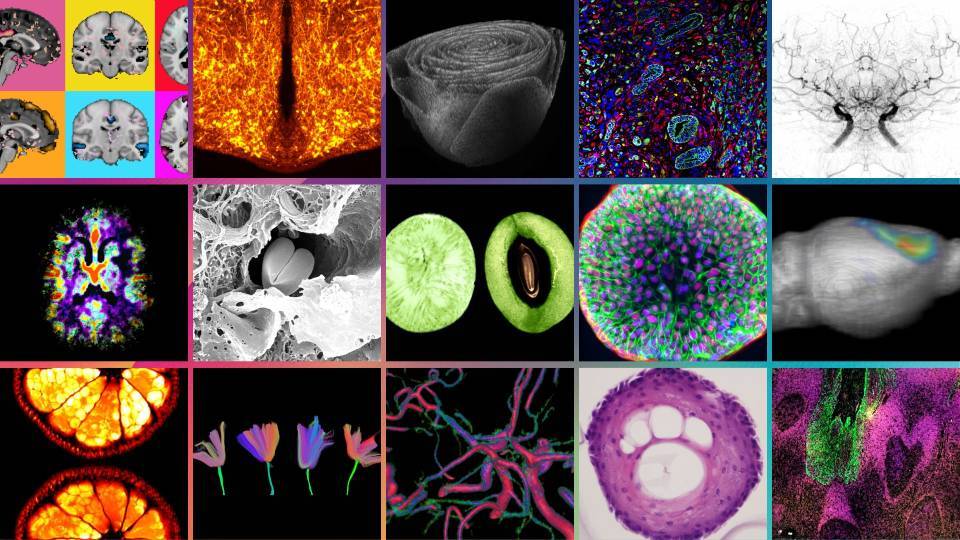
Immunofluorescence image of a pancreatic islet. Intact beta cells (red), destroyed beta cells (green) and their nuclei (blue) are observed. [Júlia Rodríguez]
Is it possible to restore insulin production?
Research on type 1 diabetes focuses mainly on determining why the immune system reacts against the pancreatic beta cells and on how to stop this attack, as well as on developing cellular therapies that make it possible to obtain functional beta cells that can be transplanted into patients. Type 2 diabetes is a multifactor disease that requires a genetic approach to metabolic and inflammatory mechanisms in order to obtain the full picture and understand what is going on. Furthermore, diet and lifestyle also play a major role in the development of the disease. This is why work is being done on prevention and on educating the population.
We do not currently have an effective cure for diabetes. “Administering insulin makes it possible to allow the patient to live with the disease but it does not restore the function of the pancreas; it merely replaces it”, points out Rosa Gasa, a researcher in the IDIBAPS group Translational research on diabetes, lipids, and obesity, led by Josep Vidal.
Insulin production is affected in both type 1 and type 2 diabetes. In type 1, however, there is a lack of insulin from the onset of the disease, whereas in the initial stages of type 2, there is an excess, which finally becomes a deficit. “This is due to what happens to the beta cells in the pancreas in each of these two types of diabetes”, says Gasa. “The beta cells, located in the pancreatic islets, are responsible for producing and secreting insulin. In type 1 diabetes, the autoimmune reaction almost completely destroys these cells. In type 2 diabetes, however, the beta cells produce insulin, but the body’s tissues become resistant and do not respond to the hormone. This causes the pancreatic islets to increase secretion. However, in the long term, this increased effort causes dysfunction of the tissue”.
Vidal and Gasa’s team is investigating how to regenerate the pancreatic beta cells. Specifically, it is looking for factors that make it possible to expand the few cells that remain. “Even in the pancreas of patients with type 1 diabetes, analyzed after death, beta cells have been found. This tells us that, somehow, despite the destruction, the tissue is trying to regenerate”. The expansion of beta cells occurs during the first year of life in humans and during lactation in mice. Studying this neonatal period in rodents has allowed Vidal-Gasa’s group to identify a protein secreted by one tissue, Wisp1, that may be key to the development of the pancreas. “This molecule also increases in babies and falls off in adults, when growth of the skeleton stops. It is probable that there are other factors that also play a role in the expansion of beta cells. Finding them may lead to major progress in regenerating the remaining pancreatic tissue”.
At the same time, Vidal and Gasa’s team is working on an area of research aimed at creating beta cells from skin cells. “The process consists of taking differentiated cells, such as fibroblasts in the dermis, and reprogramming them directly to express genes for another different cell type”, explains Gasa. “Today, just by introducing five transcription factors, proteins that bind to DNA and regulate gene expression, we can reprogram fibroblasts to produce and release human insulin in response to an increase of glucose in the blood”. In mice, this secretion of insulin can be observed up to 30 days after transplant. “This tells us that, throughout this period, the reprogramming is maintained and the cells do not die”.
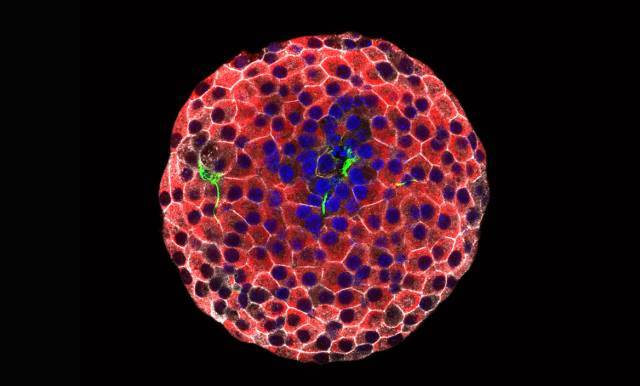
Immunofluorescence image of pancreatic islet cells in culture (red), surrounded by fibroblasts (green). Cell nuclei are stained blue. [Juan Moreno]
The advantage of this method is that transplants can be performed with a small skin sample from the same person, which reduces the risk of rejection. “Transplanting an entire pancreas is a highly complex option that is used when there is no alternative. Another option is to implant pancreatic islets from donors. The problem is that the isolation process occurs post-mortem, is inefficient, and requires between two and three donors for each patient. The patient also requires immunosuppressant treatment for life. Finally, in the case of type 1 diabetes, while 80% of transplant patients can forego insulin injections during the first year, after five years, a large part of the implanted cells has died, and a new transplant is needed. This is not a viable cure in the long term”, says the researcher.
Protecting the beta cells
Joan-Marc Servitja agrees about the importance of the therapeutic potential of transplants. “Nevertheless, the fact is that if we cannot stop the destruction of the cells, we again have a treatment that allows the patient to live with the disease but does not cure it, as the cells have to be replaced constantly”. To protect the beta cells, the group of Novials and Servitja has evaluated the use of molecules with anti-inflammatory properties, such as alpha-1 antitrypsin. “This one of the few projects we have in type 1 diabetes, as our research is more focused on type 2 diabetes, but we have had promising results. These compounds inhibit the action of the cytokines secreted by the immune system and, therefore, the attack on the beta cells. They also produce very few adverse effects”.
“We hope to move these studies forward in patients”, says Novials. “However, we often intervene in very late stages of the disease, which makes it difficult to protect and regenerate the pancreas. This is why one of our main areas of research is biomarkers. Molecules that allow us to detect pre-diabetic patients. In fact, we have identified specific small RNA molecules, or micro-RNA, in a population with slightly elevated levels of glucose in blood, but who have not yet developed type 2 diabetes”.
The study of this micro-RNA circulating in the blood inside vesicles called exosomes highlights another aspect of diabetes: its complexity. Although the pancreas is the focus of a large part of the research, other organs and tissues, such as the liver, muscle, adipose tissue, and the brain, play a role in the development of the disease. Communication between them may explain the metabolic disorder that characterizes diabetes. “The pancreas and the brain have a lot in common”, says Servitja. “In type 2 diabetes, deposits of a peptide called amylin form on the pancreatic tissue. This is a very similar process to the one that occurs in Alzheimer, where plaques of the beta-amyloid peptide accumulate on the neurons and kill them”, continues Novials. Diabetes and Alzheimer also share a potential therapeutic target, the protein BACE2, as has been shown by the researchers in a recently published article.
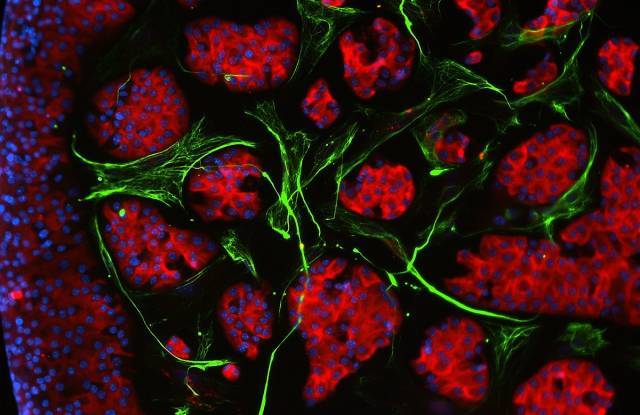
Image obtained by electron microscopy of exosomes, extracellular vesicles that contain proteins, as well as DNA and RNA molecules, and transport them through the bloodstream. [Vicent Ribas]
In nonpathologic conditions, amylin is secreted together with insulin but for reasons that we still do not understand, it folds incorrectly and accumulates, which leads to the destruction of the beta cells. “Our model of type 2 diabetes uses mice that overexpress amylin. We also fatten them so that their characteristics are more similar to those of the patients. These animals have allowed us to find therapeutic targets to regain the function of the beta cells”, says Servitja.
Another area of research that makes it possible to improve diabetes is exercise. “Lifestyle is very important. In the case of type 2 diabetes, our group has seen that exercise improves the metabolism and normalizes levels of micro-RNA that contribute to pancreatic stress. Nevertheless, patients must be given the guidelines that are most appropriate for their disease. For example, in people with type 1 diabetes, prolonged exercise sessions can cause hypoglycemia—major decreases in glucose. On the other hand, we have shown that high-intensity interval training, HIIT, has a lower risk of causing hypoglycemia”, says Novials.
“It is likely that the cure for diabetes will require the use of personalized medicine, adapted to each patient, and combined therapies that protect and regenerate the tissue. And to achieve this, multidisciplinary research with the participation of researchers from different field is essential”, concluded Gasa.
This content has been writen with the support of the Spanish Foundation for Science and Technology (FECYT).