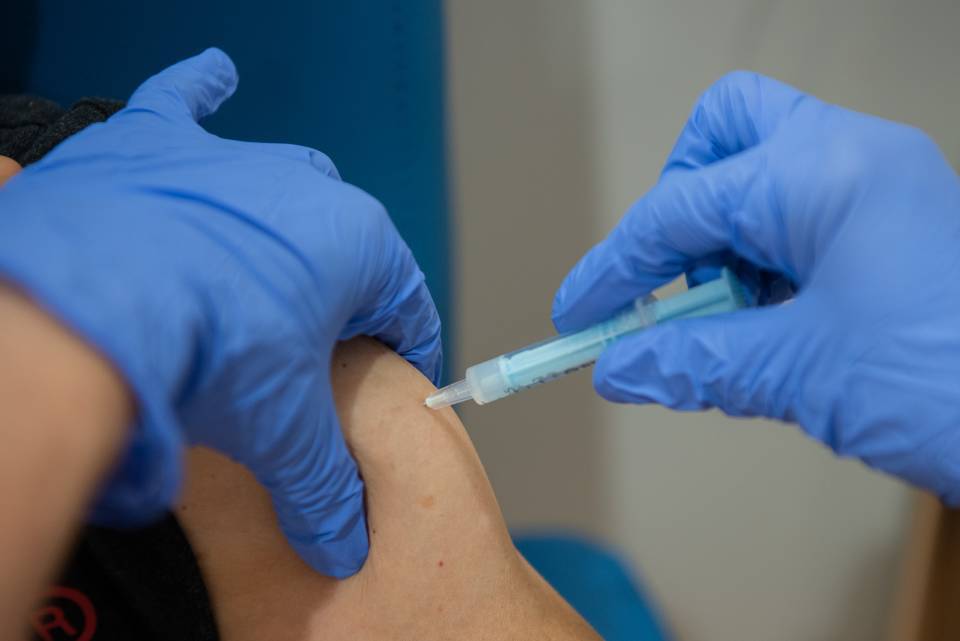
The volunteers will receive four doses of the new investigational vaccine and they will be monitored for two years in order to evaluate its safety and the volunteers’ response (immunity).
This study was assessed and approved by the Ethics Committee of the Hospital Clínic de Barcelona and the Spanish Agency of Medicines and Medical Devices (AEMPS). It will start in March/April 2023.
The volunteers will be paid.
If you are interested, please contact:
Telephone: 93 2275574 (from 9 am to 3 pm)